A white blood cell (WBC) count within the “normal” range is typically seen as reassuring.
But multiple large-scale studies tell a different story—one that challenges a central assumption in modern medicine.
Elevated white blood cell levels—even those within the so-called normal range—are consistently associated with increased mortality risk.
WBCs are a frontline marker of immune activity. When they’re persistently high, it’s not a sign of robust health, but a signal of chronic, low-grade inflammation in the body.
In the U.S., the “normal” range spans roughly 3,500–11,000 cells per microliter. But that wide range is statistical—not biological. It’s built on population averages, not on what actually supports longevity or low disease risk.
➤ What does the evidence show?
Epidemiological studies consistently find that WBC counts above ~6,000 cells/μL—well within the “normal” range—are linked to a significantly higher risk of death, especially from cardiovascular, metabolic, and cancer-related causes.
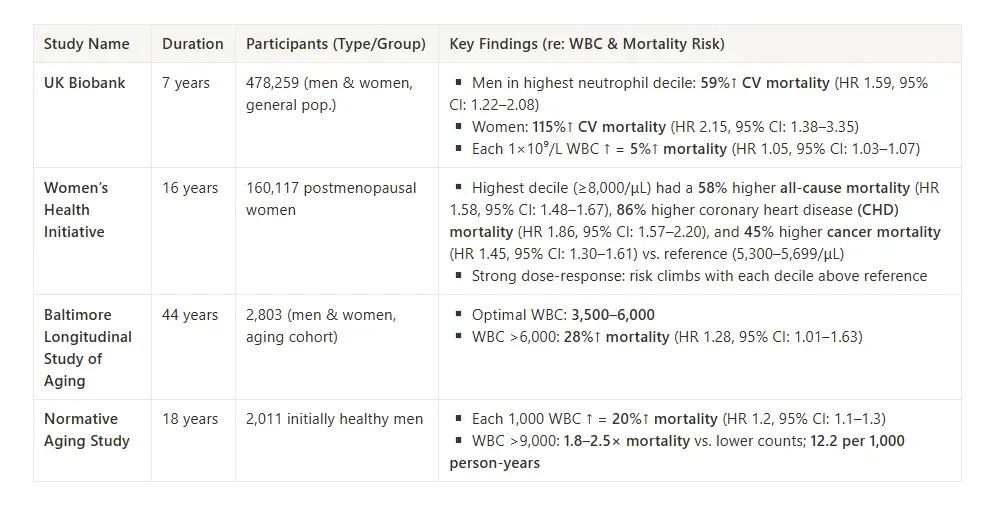
In the UK Biobank (n = 478,259), each 1×10⁹/L increase in WBC raised overall mortality risk by 5% (HR 1.05). But the risk wasn't distributed evenly:
Women in the highest neutrophil decile had more than double the risk of cardiovascular mortality (HR 2.15) compared to mid-range values.
Men in the same decile saw a 59% increase in cardiovascular mortality (HR 1.59).
Crucially, many of these elevated WBC counts still sat comfortably inside the standard reference range.
The Women’s Health Initiative (n = 160,117, postmenopausal women) found similar results over 16 years of follow-up. WBC counts ≥8,000/μL were associated with:
58% higher all-cause mortality (HR 1.58)
86% higher coronary heart disease (CHD) mortality (HR 1.86)
45% higher cancer mortality (HR 1.45)
And again, the risk increased progressively across deciles—not just at the extremes.
The Baltimore Longitudinal Study of Aging (n = 2,803, aging adults) mapped this even more precisely. It identified an optimal WBC range of 3,500–6,000 cells/μL, where mortality risk was lowest. Above this zone, mortality rose by 28% (HR 1.28).
Finally, the Normative Aging Study (n = 2,011 initially healthy men) found that:
Each 1,000-cell increase in WBC translated to a 20% rise in mortality (HR 1.2)
WBC counts >9,000 were linked to 1.8–2.5× higher mortality rates, depending on the comparison group
Across all four studies, the takeaway is the same:
Mortality risk doesn’t start when WBC exceeds 11,000—it starts rising before that, often above 6,000.
And the climb is dose-dependent, consistent, and replicable across large datasets.
➤ Why does this happen? Elevated WBC is an immune response—not a strength signal.
White blood cells rise in response to injury, infection, or cellular damage.
This is adaptive in the short term.
But when WBC remains elevated without a clear trigger, it usually points to persistent, low-grade immune activation—a state often referred to as inflammaging in the research literature.
The underlying biology is subtle, but important: chronically raised WBC reflects excess cytokine signaling, overproduction of neutrophils and monocytes, and a persistent pro-inflammatory shift in immune balance. Over time, this state promotes oxidative stress, impairs endothelial function, and accelerates tissue injury—even in people who appear outwardly healthy.
CRP (C-reactive protein) is often measured as an inflammation marker, but WBC is just as revealing—and can be tracked easily over time. In many studies, WBC correlates tightly with both CRP and increased risk for downstream diseases.
If your WBC trends above 6,000—especially alongside other signals of inflammation—it's an early indicator that your immune system is operating in a chronically low-grade activated state, and long-term risk may be accumulating.
➤ So what can you do if we're already seeing those shifts?
This is exactly where conventional medicine leaves us without guidance.
We're not acutely ill.
Our labs don't raise clinical flags.
But we're seeing subtle shifts—like a WBC trending toward 7,000—that suggest the immune system may be operating in a persistently activated state.
There's no diagnosis, no treatment protocol, and no physician playbook for this range. But the long-term signal is still there—and so is the opportunity to change course.
This is where understanding your data matters more than waiting for a diagnosis.
So where does that leave you?
You don't need a full diagnostic workup to take action.
But you can benefit a framework for understanding where your immune activity sits on the spectrum—and what might be driving it.
Here are some questions specifically designed for tracking personal baselines:
How has your WBC trended over the past 2-3 lab draws?
Was this test drawn under similar conditions as your previous ones (timing, exercise, stress level)?
Are other inflammatory markers moving in the same direction as your WBC?
What lifestyle factors have shifted since your last stable baseline?
These patterns are often missed because the WBC still falls within range. But if your goal is healthy aging, what matters isn't just the range—it's your personal trajectory.
The challenge?
Most people don't have a systematic way to understand these patterns or know when subtle changes warrant attention.
That’s why I built the Vault Chronic Inflammation & Early Aging Risk Reduction System — Know If Your Inflammation Is a Problem (& Act on It Before Disease): A Self-Guided Tool for Understanding and Adjusting Chronic Inflammation Risk.
It’s not a diagnostic tool—and it’s not designed to treat illness.
It’s a decision-support map:
A way to track WBC shifts over time, reflect on the lifestyle factors that may be driving them, and make evidence-informed changes—before inflammation becomes persistent and accelerates biological aging.
Inside, you’ll find a clear framework to translate your WBC result into an actionable zone.
Here is the Vault Chronic Inflammation & Early Aging Risk Reduction Roadmap—the resource that fills this gap, helping you translate "normal" lab values into meaningful next steps for catching and addressing low-grade inflammation before it accelerates tissue aging:
If you would like to Access the Vault Chronic Inflammation & Early Aging Risk Reduction Roadmap for Stubborn Inflammation & Lipid Markers as a one-time option (without an ongoing subscription), you can access that here:
You shouldn’t have to wait for a diagnosis to take action. With the Vault Chronic Inflammation & Early Aging Risk Reduction System, you won’t.